How To Draw Organic Molecules -are Ch4 Groups Just Lines?
Structure of Organic Molecules
- Folio ID
- 844
Here you will learn how to understand, write, draw, and talk-the-talk of organic molecules. Why were different drawing techniques developed? Organic molecules can become complicated and large. It is a tedious to take to constantly draw out every detail, especially when not necessary, so the o-chemist of the by adult these techniques to make information technology more user-friendly and easy. In add-on, some of these shorthand means of drawing molecules requite united states of america insight into the bail angles, relative positions of atoms in the molecule, and some eliminate the numerous hydrogens that tin can get in the way of looking at the backbone of the construction.
Introduction
Find the post-obit drawings of the structure of Retinol, the most common form of vitamin A. The first cartoon follows the directly-line (a.k.a. Kekul é ) structure which is helpful when you want to look at every unmarried atom; however, showing all of the hydrogen atoms makes it difficult to compare the overall structure with other similar molecules and makes it difficult to focus in on the double bonds and OH group.
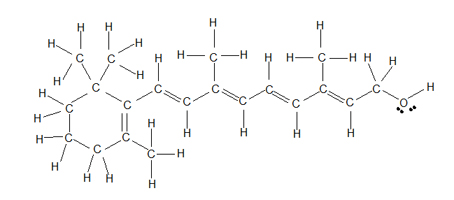
Retinol: Kekul é direct-line drawing
The following is a bond-line (a.chiliad.a. zig-zag) formula for retinol. With this simiplified representation, ane tin hands see the carbon-carbon bonds, double bonds, OH group, and CH3 groups sticking off of the the main ring and concatenation. Also, information technology is much quicker to draw this than the i to a higher place. Y'all will acquire to capeesh this blazon of formula writing after drawing a countless number of organic molecules.
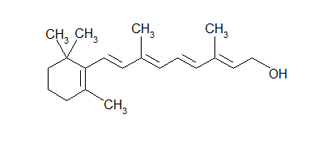
Retinol: Bond-line or zig-zag formula
Importance of Structure
Learning and practicing the basics of Organic Chemistry volition help you immensely in the long run as you learn new concepts and reactions. Some people say that Organic Chemistry is like another language, and in some aspects, it is. At first it may seem difficult or overwhelming, simply the more you exercise looking at and drawing organic molecules, the more familiar you will go with the structures and formulas. Another good idea is to get a model kit and physically make the molecules that you take trouble picturing in your head.
Through general chemical science, you may have already experienced looking at molecular structure. The different ways to draw organic molecules include Keku l é (straight-line), Condensed Formul as, and Bond-Line Formulas (zig-zag). It will be more helpful if yous become comfortable going from one style of cartoon to another, and look at drawings and understanding what they hateful, than knowing which kind of drawing is named what.
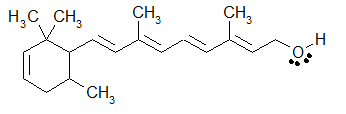
An example of a cartoon that incorporates all three means to draw organic molecules would exist the post-obit additional drawing of Retin ol. The majority of the drawing is Bond-line (zig-zag) formula, merely the -CHthree are written as condensed formulas, and the -OH group is written in Kekul é course.
A widely used way of showing the 3D structure of molecules is the use of dashes, wedges, and straight lines. This drawing method is essential because the placement of dissimilar atoms could yield different molecules even if the molecular formulas were exactly the aforementioned. Beneath are two drawings of a 4-carbon molecule with ii chlorines and 2 bromines attached.
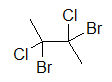
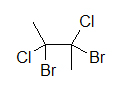
4-carbon molecule with 2 chlorines and 2 bromines iv-carbon molecule with 2 chlorines and 2 bromines
Both drawings look similar they represent the same molecule; however, if we add dashes and wedged we will come across that two different molecules could be depicted:
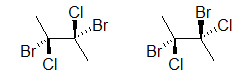
The two molecules above are unlike, prove this to yourself past building a model. An easier manner to compare the 2 molecules is to rotate one of the bonds (here, it is the bond on the correct):
.jpg?revision=1&size=bestfit&width=246&height=74)
Notice how the molecule on the right has both bromines on the same side and chlorines on the same side, whereas the get-go molecule is different. Read almost Dashed-Wedged Line structures, bottom of folio, to sympathise what has been introduced higher up. You lot will learn more about the importance of atomic connectivity in molecules as yous continue on to learn most Stereochemistry.
Cartoon the Structure of Organic Molecules
Although larger molecules may look complicated, they can be easily understood past breaking them downwardly and looking at their smaller components.
All atoms desire to have their valence beat out full, a "closed beat out." Hydrogen wants to have 2 due east- whereas carbon, oxygen, and nitrogen desire to accept 8 e-. When looking at the different representations of molecules, keep in mind the Octet Rule. Also remember that hydrogen tin can bond 1 time, oxygen tin bail upwardly to two times, nitrogen can bond upward to three times, and carbon can bond upwardly to four times.
.jpg?revision=1&size=bestfit&width=720&height=104)
Kekulé (a.thou.a. Straight-Line Structures)
Kekul é structures are similar to Lewis Structures, merely instead of covalent bonds being represented by electron dots, the two shared electrons are shown by a line.
(A) 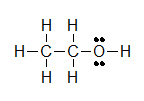 (B)
(B)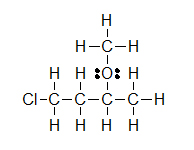 (C)
(C)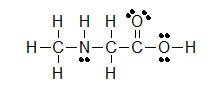
Lone pairs remain equally two electron dots, or are sometimes left out fifty-fifty though they are still in that location. Observe how the three solitary pairs of electrons were non draw in around chlorine in example B.
Condensed Formulas
A condensed formula is made up of the elemental symbols. The lodge of the atoms suggests the connectivity. Condensed formulas can be read from either direction and HiiiC is the aforementioned as CHthree, although the latter is more common because Expect at the examples below and match them with their identical molecule under Kekul é structures and bond-line formulas.
(A) CH3CHtwoOH (B) ClCH2CHtwoCH(OCH3)CH3 (C) H3CNHCH2COOH
Let's look closely at example B. Every bit you lot go through a condensed formula, you lot want to focus on the carbons and other elements that aren't hydrogen. The hydrogen's are important, but are usually there to complete octets. Also, notice the -OCH3 is in written in parentheses which tell y'all that it not role of the primary chain of carbons. Every bit you read through a a condensed formula, if you lot reach an cantlet that doesn't accept a complete octet past the time you reach the next hydrogen, so it'south possible that in that location are double or triple bonds. In example C, the carbon is double bonded to oxygen and single bonded to another oxygen. Notice how COOH ways C(=O)-O-H instead of CHiii-C-O-O-H considering carbon does not have a complete octet and oxygens.
Bail-Line (a.k.a. zig-zag) Formulas
The proper name gives away how this formula works. This formula is full of bonds and lines, and considering of the typical (more stable) bonds that atoms tend to make in molecules, they frequently cease upward looking like zig-zag lines. If yous work with a molecular model kit you lot volition find information technology difficult to make stick straight molecules (unless they contain sp triple bonds) whereas zig-zag molecules and bonds are much more than viable.
(A) 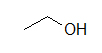 (B)
(B)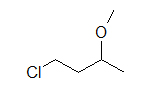 (C)
(C)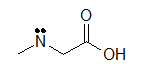
These molecules correspond to the exact same molecules depicted for Kekul é structures and condensed formulas. Notice how the carbons are no longer drawn in and are replaced by the ends and bends of a lines. In addition, the hydrogens have been omitted, only could be easily fatigued in (see practice problems). Although we exercise not unremarkably draw in the H's that are bonded to carbon, we do draw them in if they are connected to other atoms besides carbon (instance is the OH group above in instance A) . This is done because it is not ever articulate if the not-carbon atom is surrounded by lone pairs or hydrogens. Also in example A, discover how the OH is drawn with a bail to the second carbon, just it does not mean that there is a third carbon at the end of that bail/ line.
Dashed-Wedged Line Construction
As you may take guessed, the Dashed-Wedged Line construction is all nigh lines, dashes, and wedges. At first it may seem confusing, but with practice, understanding dash-wedged line structures will get similar second nature. The following are examples of each, and how they can be used together.
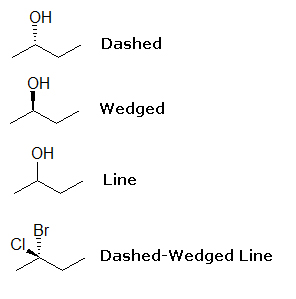
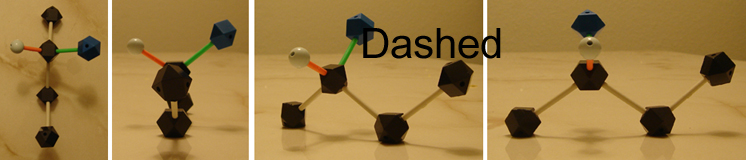
Above are 4-carbon chains with attached OH groups or Cl and Br atoms. Retrieve that each line represents a bail and that the carbons and hydrogens have been omitted. When you wait at or describe these structures, the straight lines illustrate atoms and bonds that are in the same plane, the aeroplane of the paper (in this example, computer screen). Dashed lines show atoms and bonds that get into the page, behind the airplane, away from you. In the above example, the OH group is going into the plane, while at the aforementioned time a hydrogen comes out (wedged).
Bluish dewdrop= OH grouping; White bead=H
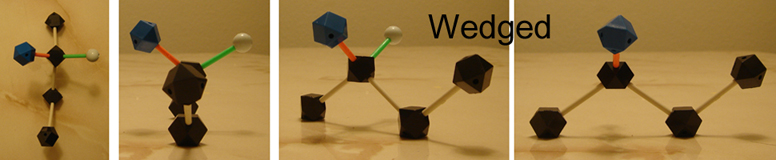
Wedged lines illustrate bonds and atoms that come out of the page, in front of the plane, toward yous. In the 2nd diagram above, the OH group is coming out of the plane of the paper, while a hydrogen goes in (dashed).
Blue dewdrop= OH group; White dewdrop=H
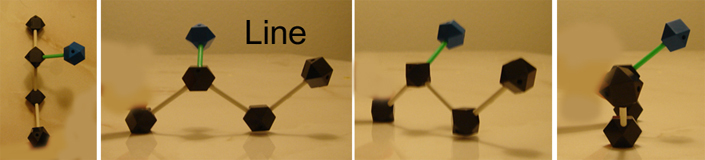
As stated before, straight lines illustrate atoms and bonds that are in the same plane as the paper, but in the 2d instance, the straight line bail for OH means that it it unsure or irrelevant whether OH is going abroad or toward you. It is also causeless that hydrogen is also connected to the same carbon that OH is on.
Bluish bead= OH group; H is not shown
Endeavor using your model kit to see that the OH grouping cannot lie in the same plane at the carbon chain (don't forget your hydrogens!). In the final 2Dexample, both dashed and wedged lines are used because the fastened atoms are not hydrogens (although dashed and wedged lines can be used for hydrogens).The chlorine is coming out the page while bromine is going into the page.

Blue bead=Cl; Red bead=Br
References
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry: Construction and Part. 5th ed. New York: Westward. H. Freeman Company, 2007. 38-twoscore.
- Klein, David R. Organic Chemistry I As a Second Linguistic communication. 2d ed. Hoboken, NJ: John Wiley & Sons, Inc, 2007. 1-14.
Contributors
- Choo, Ezen (2009, UCD '11)
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Fundamentals/Structure_of_Organic_Molecules
Posted by: kingassfor.blogspot.com


0 Response to "How To Draw Organic Molecules -are Ch4 Groups Just Lines?"
Post a Comment